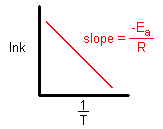Temperature
Dependence of the Rate of a
Reaction
☝Most of the chemical reactions are accelerated by increase in temperature.
For example, in decomposition of N2O5, the time taken for half of the
original amount of material to decompose is 12 min at 50o
C, 5 h at
25o
C and 10 days at 0o
C.
☝It has been found that for a chemical reaction with rise in
temperature by 10°, the rate constant is nearly doubled.
☝The temperature dependence of the rate of a chemical reaction can
be accurately explained by Arrhenius equation .It was first
proposed by Dutch chemist, J.H. van’t Hoff but Swedish chemist, Arrhenius provided its physical justification and interpretation.
K = A e-Ea/RT
Where,
k = Rate constant A= Frequency factor
e = mathematical quantity Ea= activation energy
R = gas constant T = kelvin temperature
Activation energy
☝The minimum quantitiy of external energy required for the conversion of reactant into product or to produce an unstable intermediate is called activation energy. It is E
☝Rate of reaction is inversely proportional to the activation energy.
☝Therefore, greater value of activation energy leads to lower rate of reaction and increased influence of temperature change on the rate constant.
☝Reaction coordinates represents the profile of energy change when reactants change into product i.e. The energy profile of a chemical reaction is a representation of the most favourable energetic pathway for the reactants to be transformed into the products.
☝All the molecules in the reacting species do not have the same kinetic energy. since it is difficult to predict the behaviourof any one molecules with precision.
☝ This is due to collisions between the moving molecules so that their energies are transferred from one molecule to another.
☝ Ludwig Boltzmann and James Clark Maxwell used statistics to predict the behaviour of large number of molecules. If the energy of molecules are plotted against the corresponding fraction of molecules, NE/NT with a given energy at a particular temperature, a curve is obtained. This is called Maxwell’s distribution of energies( given by Ludwig Boltzmann and James Clark Maxwell).
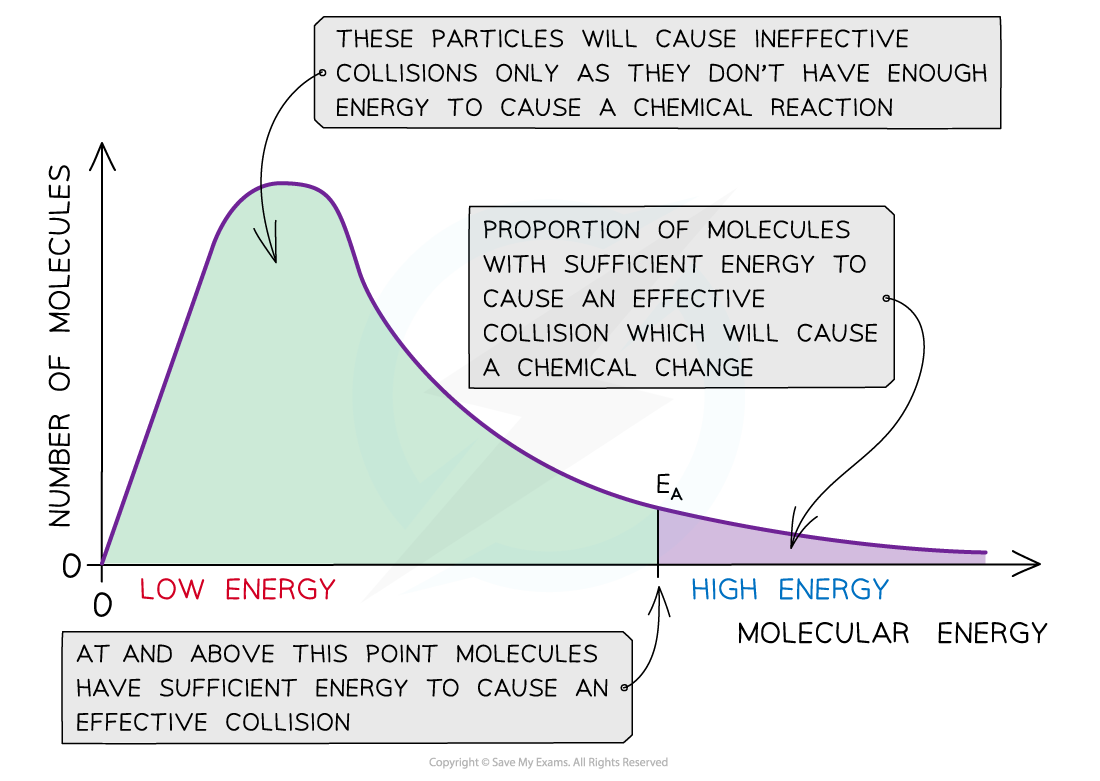
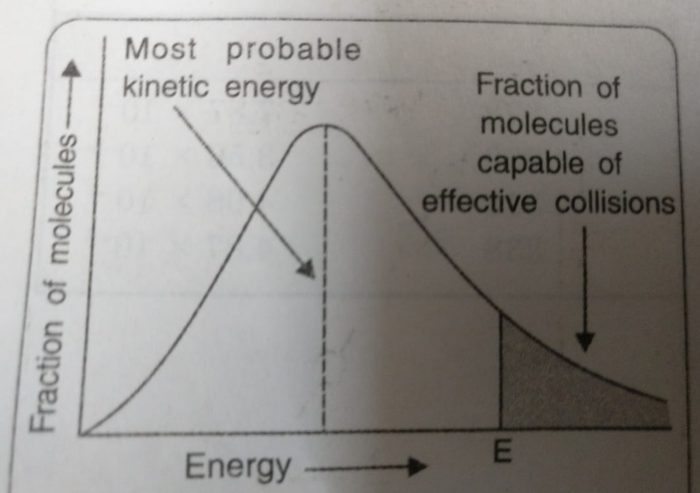
1) The fraction of molecules having very low or very high energies is very small.
2) Most of the molecules have intermediate kinetic energies as shown by the peak in the graph.
This peak corresponds to most probable kinetic energy i.e. kinetic energy of maximum fraction of molecules.
3) E corresponds to minimum or threshold energy required for effective collisions. The molecules having energy equal to or greater than E will result in the formation of products and this fraction of molecules capable of effective collisions is very small.
4) For reactions having low values of E, there will be larger fraction of colliding molecules which produce effective collisions and hence, rate of the reaction will be high.
If the value of E for a particular reaction is high, then only a few collisions will be sufficiently energetic to give products while all other collisions will be ineffective. The reaction will proceed very slowly.
Effect of increase in temperature on the number of effective collisions


a) The curve at higher temperature gets shifted towards the right indicating that at higher temperature, the molecules have higher energies.
b) The curve at higher temperature is flatter than that at lower temperature which also indicates that the number of molecules with higher energy content have increased.
c) The number of molecules possessing energies equal to or greater than E, is proportional to area abcd at temperature T1, and area abef at temperature T2. The area abef is roughly twice as large as abcd.
Since the rate of reaction depends upon the number of molecules which possess energies larger than activation energy (for effective collisions), it may be interpreted that the fraction of molecules possessing activation energy has increased approximately two times and thereby, increases the rate by two times for a rise of 10 degrees.
Increase in the rate of reaction with the rise in temperature is mainly due to the increase in number of effective collisions.
d) fraction of molecules NE/NT = e-Ea/RT
Arrhenius equation
- The formula used to calculate the energy of activation and justify the effect of temperature on rate of reaction is called Arrhenius Equation.
- It is given by the formula,
K = A e-Ea/RT
Where,
k = Rate constant A= Frequency factor
e = mathematical quantity Ea= activation energy
R = gas constant T = kelvin temperature
☝The factor e-Ea/RT corresponds to the fraction of molecules that have the kinetic energy greater than Ea.
Thus, it has been found from Arrhenius equation that increasing the temperature or decreasing the activation energy will result in an increase in the rate of the reaction and an exponential increase in the rate constant.
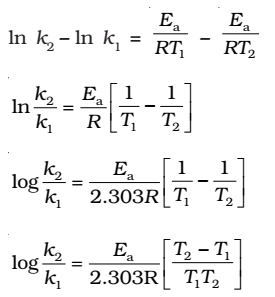
Taking natural logarithm of both sides of equation

The plot of ln k vs 1/T gives a straight line with
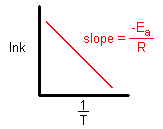
(since A is constant for a given reaction)
k1and k2 are the values of rate constants at temperatures T1 and T2 respectively.
Subtracting equation form, we obtain
Effect of
Catalyst
☝ A catalyst is a substance which increases the rate of a reaction without
itself undergoing any permanent chemical change
2KClO3 -----> 2 2 KCl + 3O2 (MnO2 as catalyst)
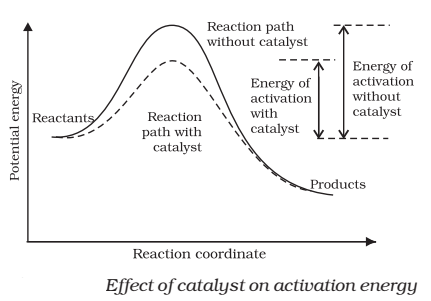
characteristics of catalyst-
☝catalyst provides an
alternate pathway or reaction mechanism by
reducing the activation energy between
reactants and products
☝ it lower the value of activation energy, faster
will be the rate of a reaction.
☝A small amount of the catalyst can catalyse
a large amount of reactants.
☝A catalyst does
not alter Gibbs energy, ΔG of a reaction.
☝It
catalyses the spontaneous reactions but does not catalyse nonspontaneous reactions.
☝It is also found that a catalyst does not change
the equilibrium constant of a reaction rather, it helps in attaining the
equilibrium faster, that is,
☝it catalyses the forward as well as the backward
reactions to the same extent so that the equilibrium state remains same
but is reached earlier.
Collision Theory
According to this theory,
the reactant molecules are
assumed to be hard spheres and reaction is postulated to occur when
molecules collide with each other.
👍the rate of chemical
reactions depends on
a. activation energy
b.collision frequency (Z)- The number of collisions per second per unit volume of the reaction mixture is known as collision frequency (Z)
For a
bimolecular elementary reaction
A + B → Products
rate of reaction can be expressed as
Rate = ZAB e -Ea/RT
c.Steric or probability factor (P)-The proper orientation of
reactant molecules lead to bond
formation whereas improper
orientation makes them simply
bounce back and no products are
formed.
To account for effective collisions,molecules
must be properly oriented i.e.
Rate = PZAB e -Ea/RT
Next Collision Theory