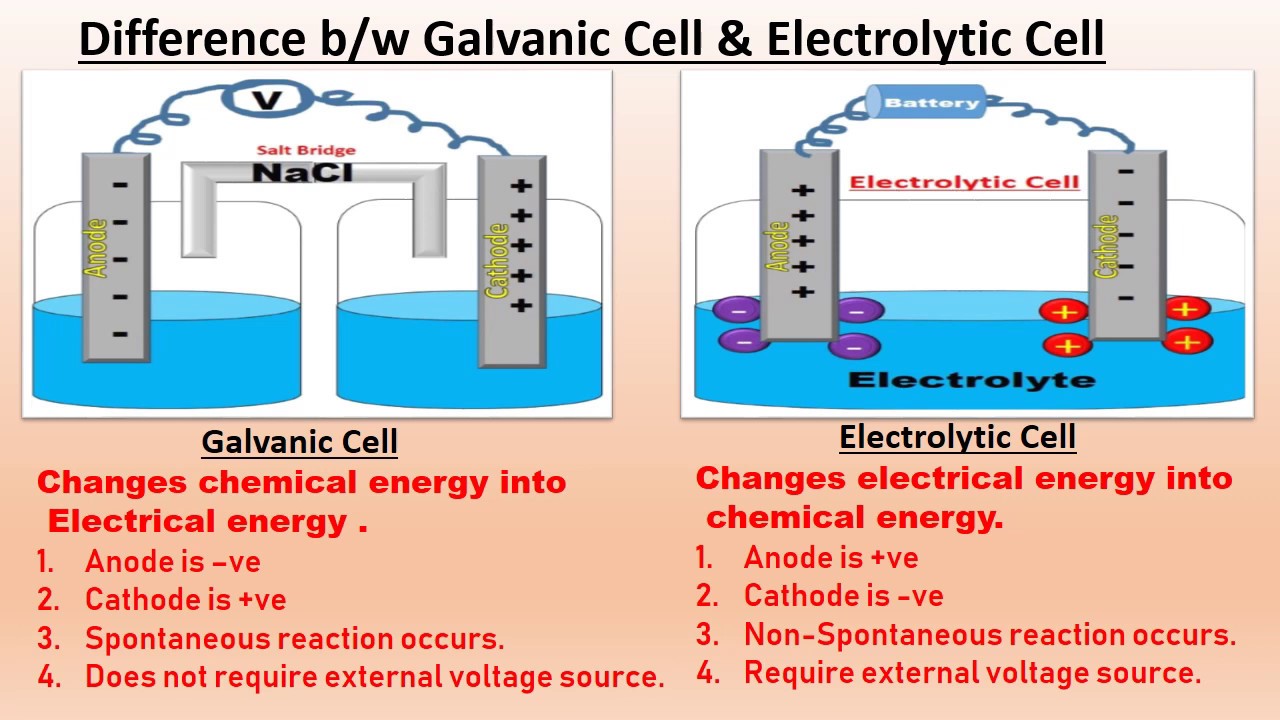
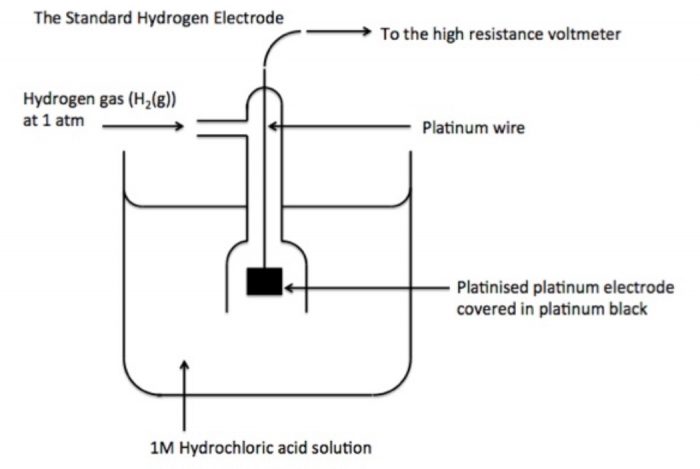
1. What is the principle of volumetric analysis?
Answer. In volumetric analysis, the concentration of a solution is determined by
allowing a known volume of the solution to react, quantitatively with another
solution of known concentration.
2. What is titration ?
Answer. The process of adding one solution from the burette to another in the
conical flask in order to complete the chemical reaction involved, is known as
titration.
3. What is a standard solution ?
Answer. A solution whose strength is known is called a standard solution.
4. What is a normal solution ?
Answer. A solution containing one gram-equivalent mass of the solute per litre of the
solution is called a normal solution.
5. What is indicator ?
Answer. Indicator is a chemical substance which changes colour at the end point
6. What is end point ?
Answer. The stage during titration at which the reaction is just complete is known as
the end point of titration.
7. Why a titration flask should not be rinsed ?
Answer. This is because during rinsing-some liquid will remain sticking to the titration
flask therefore the pipetted volume taken in the titration flask will increase.
8. What are primary and secondary standard substances?
Answer. A substance is known as primary standard if it is available in high degree of
purity, if it is stable and unaffected by air, if it does not gain or lose moisture in air, if
it is readily soluble and its solution in water remains as such for long time.
On the other hand, a substance which does not possess the above characteristics is
called a secondary standard substance. Primary standards are crystalline oxalic add,
anhydrous Na2CO3 , Mohr’s salt, etc.
9. Burette and pipette must be rinsed with the solution with which they are filled,
why ?
Answer. The burette and pipette are rinsed with the solution with which they are
filled in order to remove any water sticking to their sides, which otherwise would
decrease the cone, of the solutions to be taken in them
10.It is customary to read lower meniscus in case of colourless and transparent
solutions and upper meniscus in case of highly coloured solutions, why ?
Answer. Because it is easy to read the lower meniscus in case of colourless solutions,
while the upper meniscus in case of coloured solutions. In case of coloured solutions
lower meniscus is not visible clearly.
11.What is a molar solution ?
Answer. A molar solution is a solution, a litre of which contains one gm-mole of the
substance. This is symbolised as 1M.
12.Why the last drop of solution must not be blown out of a pipette?
Answer. Since the drops left in the jet end is extra of the volume measured by the
pipette.
13.Pipette should never be held from its bulb, why ?
Answer. The body temperature may expand the glass and introduce an error in the
measurement volume.
14. What is permanganometry ?
Answer. Redox titrations involving KMnO4 as the oxidising agent are called
permanganometric titrations.
15.Which is an oxidising agent and a reducing agent in the reaction between
KMnO4 and FeSO4?
Answer. KMnO4 acts as oxidising agent and FeSO4 acts as reducing agent.
16.What is the indicator used in KMnO4 titration ?
Answer. No indicator is used because KMnO4 acts as a self-indicator
17.Why does KMnO4 act itself as an indicator ?
Answer. In the presence of dilute sulphuric acid, KMnO4 reacts with reducing agent
(oxalic acid or . ferrous sulphate). When all the reducing agent has been oxidised, the
excess of KMnO4 is not decomposed and imparts pink colour to the solution.
18.What is the end point in KMnO4 titrations ?
Answer. From colourless to permanent light pink.
19.Why is Mohr’s salt preferred as a primary standard over ferrous sulphate in
volumetric
analysis ?
Answer. This is because of the fact that Mohr’s salt is stable and is not readily
oxidised by air. Ferrous sulphate gets oxidised to ferric sulphate.
20. Why are a few drops of dilute sulphuric acid added while preparing a standard
solution
of Mohr’s salt ?
Answer. Few drops of H2SO4 are added to prevent the hydrolysis of ferrous sulphate.
21.Why a burette with rubber pinch cock should not be used in KMnO4 titrations ?
Answer. Because KMnO4 attacks rubber.
22.Sometimes a brown ppt. is observed in KMnO4 titrations. Why ?
Answer. It is due to insufficient quantity of dil. sulphuric acid. Brown coloured ppt.
(MnO2.H20) is formed due to the incomplete oxidation of KMnO4.
23.Why should you heat the oxalic acid solution to about 60-70°C before titrating with
KMnO4 solution ?
Answer. In cold, the reaction is very slow due to the slow formation of Mn
2+
ions.
Oxalic acid is heated to speed up the liberation of Mn
2+
ions which then autocatalyses
the reaction and thus the reaction proceeds rapidly. This also serves the purpose of
expelling the carbondioxide evolved during the reaction which otherwise does not
allow the reaction to go to completion.
24.What is the equivalent mass of KMnO4 when it acts as oxidizing agent in acidic
medium ?
Answer. KMnO4 loses 5 electrons per molecule, when it acts as oxidizing agent in the
presence of acids. Therefore, its equivalent mass is one-fifth of its molecular mass.
25.Are ‘molality’ and “molarity’’ same ?
Answer. No, molality of a solution is defined as the number of moles of solute
present in 1000 grams of the solution whereas molarity tells us about the number of
moles of the solute present per litre of the solution.
26.What is the basicity of H2SO4 ?
Answer. 2.