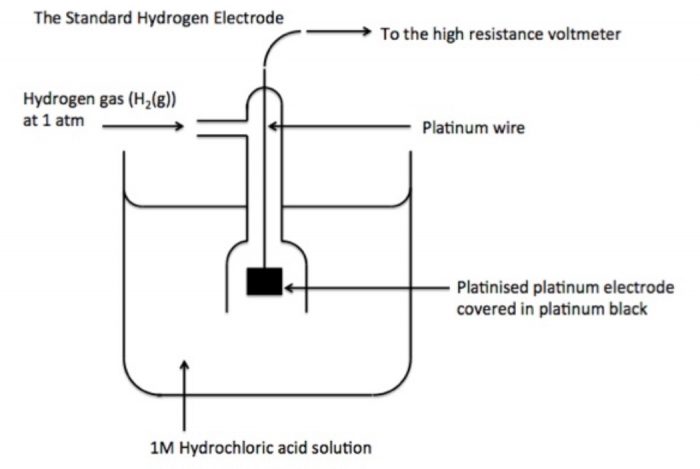
ü It is reference electrode consists of a platinum electrode(Pt wire fitted in glass tube) in contact with H2 gas (1 atm) and aqueous H+ ions (1 M).
ü It is assigned 0.0 V electrode potential.
ü It may behave as anodic or cathodic half cell.
ü It is represented as
Pt(s)|H2(g)(aH2= 1)|H+(aq)(aH+ = 1).
ü When SHE is coupled with an other half cell then cell potential is the value of the electrode potential of half cell.

No comments:
Post a Comment
If you have any doubts, Please let me know.