Vapour pressure of liquid solution:-
when a liquid solution is kept in a closed when a container, some of the molecules of liquid from its surface starts evaporating & start accumulating in the space above the surface of the liquid. due to lesser force of attraction and more kinetic energy present in surface molecule.
Molecules of liquid in the vapour phase more randomly & strike the liquid surface & get condensed. The process keeps on until equilibrium between evaporation & condensation reached.
The pressure exerted by molecules of vapour phase present in the vacant space of the container over the liquid surface is called vapour pressure.
Factors on which vapour pressure of a liquid depends : -
( a). Nature of the liquid - Each liquid has a characteristics vapour pressure because each liquid has different magnitude of intermolecular forces .
(b). Temperature - The vapour pressure of a liquid increase with increase in temperature. due to increase in kinetic energy of molecules present in liquid(with increase in temperature).
(c).Boiling point:-vapour pressure is inverse to boiling point.
(d). Purity of liquid : pure liquid has always higher vapour pressure compare to its solution .
Vapour Pressure of liquid - liquid solutions:-
The French chemist, Francois Mate Raoult (1886)gave a quantitative relationship between partial vapour pressure of each component of the solution & its mole fraction.
It states that the vapour pressure of solution is directly proportional to mole faction of a solute added to the solution
Raoult law for volatile liquids: -It states that The partial pressure of each volatile component of the solution is directly proportional to its mole fraction present in solution.
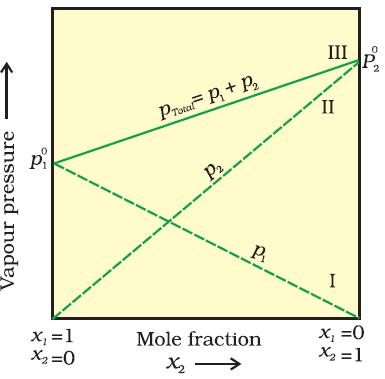
let us consider a binary solution of two volatile liquids denoted as component-1 & 2. Let the total vapour pressure be Ptotal at equilibrium state, p1 & p2 be the partial vapour pressure of two components 1 & 2 respectively.
Thus, by applying RaouIt's law for component - 1
p1 α 𝝌1 . . . . . . . . .(i)
P1 = p10 𝝌1 . . . . . . . . . . . .(ii) where p10 is the vapour pressure of pure component -1
similarly for component -2
p2 α 𝝌2. . . . . . . . .(iii)
P2 = p20 𝝌2 . . . . . . .(iv) where p10 is the vapour pressure of pure component -2
According to Dalton's law of partial pressures-
the total pressure (p total)over the solution phase will be the sum of the partial pressure of the components of the solution.
by substituting the values of p1 & p2 we get
ptotal=p10 𝝌1 + p20 𝝌2 . . . . . . . . .(vi)
= (1-𝝌2) p10+ p20 𝝌2 . . . . . . . (vii) Since 𝝌1 + 𝝌2 = 1
= p10+( p20 - p10 )𝝌2 . . . . . . .(viii)
for-composition of vapour phase in equilibrium:
Let y1 & y2mole fraction and p1,p2 partial pressure of the component 1 & 2 respectively in vapour phase.
P1 = y1 ptotal . . . . . (ix)
when a liquid solution is kept in a closed when a container, some of the molecules of liquid from its surface starts evaporating & start accumulating in the space above the surface of the liquid. due to lesser force of attraction and more kinetic energy present in surface molecule.
Molecules of liquid in the vapour phase more randomly & strike the liquid surface & get condensed. The process keeps on until equilibrium between evaporation & condensation reached.
The pressure exerted by molecules of vapour phase present in the vacant space of the container over the liquid surface is called vapour pressure.
Factors on which vapour pressure of a liquid depends : -
( a). Nature of the liquid - Each liquid has a characteristics vapour pressure because each liquid has different magnitude of intermolecular forces .
(b). Temperature - The vapour pressure of a liquid increase with increase in temperature. due to increase in kinetic energy of molecules present in liquid(with increase in temperature).
(c).Boiling point:-vapour pressure is inverse to boiling point.
(d). Purity of liquid : pure liquid has always higher vapour pressure compare to its solution .
Vapour Pressure of liquid - liquid solutions:-
The French chemist, Francois Mate Raoult (1886)gave a quantitative relationship between partial vapour pressure of each component of the solution & its mole fraction.
It states that the vapour pressure of solution is directly proportional to mole faction of a solute added to the solution
Raoult law for volatile liquids: -It states that The partial pressure of each volatile component of the solution is directly proportional to its mole fraction present in solution.
let us consider a binary solution of two volatile liquids denoted as component-1 & 2. Let the total vapour pressure be Ptotal at equilibrium state, p1 & p2 be the partial vapour pressure of two components 1 & 2 respectively.
Thus, by applying RaouIt's law for component - 1
p1 α 𝝌1 . . . . . . . . .(i)
P1 = p10 𝝌1 . . . . . . . . . . . .(ii) where p10 is the vapour pressure of pure component -1
similarly for component -2
p2 α 𝝌2. . . . . . . . .(iii)
P2 = p20 𝝌2 . . . . . . .(iv) where p10 is the vapour pressure of pure component -2
According to Dalton's law of partial pressures-
the total pressure (p total)over the solution phase will be the sum of the partial pressure of the components of the solution.
by substituting the values of p1 & p2 we get
 |
= (1-𝝌2) p10+ p20 𝝌2 . . . . . . . (vii) Since 𝝌1 + 𝝌2 = 1
= p10+( p20 - p10 )𝝌2 . . . . . . .(viii)
for-composition of vapour phase in equilibrium:
Let y1 & y2mole fraction and p1,p2 partial pressure of the component 1 & 2 respectively in vapour phase.
P1 = y1 ptotal . . . . . (ix)
P2 = y2 ptotal . . . . . .(x)
or y1= p1/ptotal y2= p2 / ptotat
y1= p1 𝝌1⟋p01 𝝌1 +p20𝝌2 . . . . . . . ( xi)
y2= p2 𝝌2/p01 𝝌1 +p20𝝌2 . . . . . . . . . xii
eq. ( xi) & (xii) may be used to find out the composition of in vapour phase.
Raoults Law for solutions containing solids in liquids:
Only liquids are volatile components in solution. So it can evaporate as vapour. Thus thus vapour pressure is due to volatile liquid.
Raoult's law may state that the partial vapour pressure of volatile component in solution is directly proportional to its mole fraction.
In mathematics
pvol α 𝝌vol
Pvol = pvol0 𝝌vol
Solid non volatile does not contribute in vapour pressure, since it does not form vapour.
y1= p1 𝝌1⟋p01 𝝌1 +p20𝝌2 . . . . . . . ( xi)
y2= p2 𝝌2/p01 𝝌1 +p20𝝌2 . . . . . . . . . xii
eq. ( xi) & (xii) may be used to find out the composition of in vapour phase.
Raoults Law for solutions containing solids in liquids:
Only liquids are volatile components in solution. So it can evaporate as vapour. Thus thus vapour pressure is due to volatile liquid.
Raoult's law may state that the partial vapour pressure of volatile component in solution is directly proportional to its mole fraction.
In mathematics
pvol α 𝝌vol
Pvol = pvol0 𝝌vol
Solid non volatile does not contribute in vapour pressure, since it does not form vapour.
Ideal and non ideal solutions:-
Ideal solution
i) Obey Raoults law P1 = p10 𝝌1 P2 = p20 𝝌2
i) Obey Raoults law P1 = p10 𝝌1 P2 = p20 𝝌2
ii) ΔH (mix) = 0 ΔV (mix) = 0
non ideal solution
i) don't obey raoults law P1 # p10 𝝌1 P2 # p20 𝝌2
ii) ΔH (mix) ≠ 0 ΔV (mix) ≠ 0
| ideal | non ideal solution |
| Independent
For Example, consider two liquids A and B, and mix them. The formed solution will experience several intermolecular forces of attractions inside it, which will be:
The solution is said to be an ideal solution, only
when the intermolecular forces of attraction
between A – A, B – B and A – B are nearly equal.
Examples of Ideal Solutions
| Interacts with others. The solute-solute and solvent-solvent interaction is different from that of solute-solvent interaction
Non-ideal solutions are of two types:
(a) Non-ideal solutions showing positive deviations: Positive deviation occurs when total vapour pressure for any mole fraction is more than what is expected according to Raoult’s law. This happens when the new interactions are weaker than the interaction in the pure component
(A – B < A – A or B – B interactions). 
It forms minimum boiling azeotropes, for example, C2H5OH + cyclohexane. The Bonding present in pure C2H5OH is cut off on adding cyclohexane. For such solution, ΔV and ΔH are positive.
Examples:

Non-ideal solutions showing negative deviations: Negative deviation occurs when total vapour pressure for any mole fraction is less than what is expected according to Raoult’s law. This happens when the new interactions are stronger than the interaction in the pure component
(A – B > A – A or B – B interactions). 
It forms maximum boiling azeotrope, for example, CHCl3+ CH3COCH3.For such solutions, ΔV and ΔH are negative.
Examples:
|
No comments:
Post a Comment
If you have any doubts, Please let me know.